Endovascular Graft Delivery
Artery Stent Graft Deployment
Often you just want a product to do its job and get out of the way. When a surgeon is operating on your damaged arteries, they have enough to think about. You want them focused on operating on you – not how to operate their tools.
When we started a conversation with Cook Medical, one of the world’s leading medical device companies, we both really wanted to respect that truth. A great surgery in progress is a sight to behold; every movement carefully considered, graceful, precise. In that context, the best medical product should almost be an invisible aid. It should only be there just as much as it needs to be, no more.
Read More
When together we started work on a new endovascular graft device to support damaged arteries, our questions started there. How could we embed the wisdom of great surgeons? What if it felt so safe the right result became almost inevitable? How could we design a device so intuitive it felt effortless? What if using it felt like being guided by hidden hands?
The Challenge
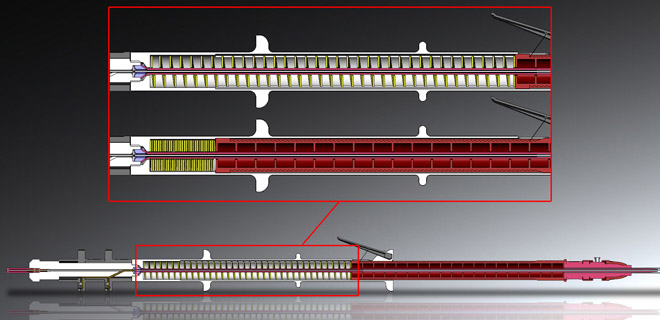
Cook asked us to help them explore the future of endovascular graft delivery devices – precision tools used to deploy stent grafts into a patient’s artery to reinforce narrowed or weakened artery walls.
Embody that research and intellectual property in the medical equivalent of a concept car – an innovative, working and production-ready prototype that fulfills the complex human and technical needs of a highly demanding surgical environment.
The Journey
Surgery to deliver an endovascular graft demands a product solution that reduces the chance of human error and provides the surgeon with great precision, control and above all, a very high level of confidence and safety. In this environment, failures can be fatal. Having a rigorous understanding of the human factors was critical. So with the help of Cook’s R&D team, that’s where our discovery process began.
How do you design a great surgery product when the surgeon never even looks at it while its in use? One of the big insights was that surgeons aren’t actually looking at the person they’re operating on during these procedures. Because endovascular procedures are done through keyhole surgery, there’s not much point looking at the patient unless you’ve got x-ray vision to see inside them. So the surgeon’s eyes are glued to an overhead monitor showing a live x-ray. Looking at the product is a dangerous distraction from taking care of the patient.
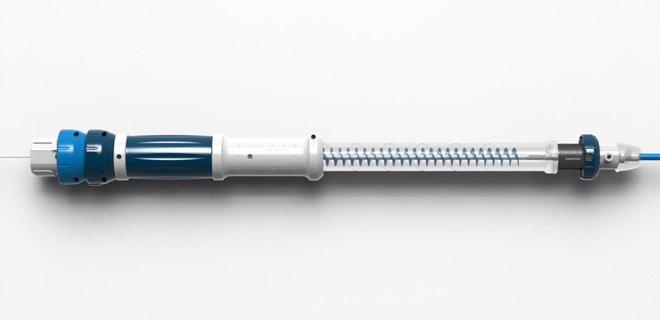
With their eyes occupied, this kind of surgery is done by touch and by sound. So we designed the device to be operated “blind”. There’s a very particular order in which the surgery has to happen – insert, move smoothly to the target area in the artery, stop, deploy the scaffolding graft, retract steadily. The surgeon needs to be able to find the each corresponding area of the product by touch, and then measure their progress by vibration and by sound. All through the thick, heavy surgical gloves which a surgeon is always wearing.
In response to this, we designed the rhythmic click of the retracting ratchet to tell the surgeon they’re progressing steadily, a sharper click to tell them they’re done. The tactile feeling of the distinct shapes and textures on different parts of the device lets them know exactly where they are and where they should be, and the device simply refuses to work if the surgeon starts using the wrong area. The delicately balanced weight of the device is light enough to let them feel the movement of the wire through the artery, but heavy enough to resist any potential twitch movements.
While the human interface issues were incredibly challenging, the technical requirements of making it manufacturable were equally challenging. It was no mean feat to balance the the technical needs of sophisticated mechanisms in very small spaces against the often conflicting human needs we were hoping to address – all in a strictly regulated environment.
Our intensive concept phase required a balance of technical and creative problem solving. We iterated through multiple ideas, pulling apart and prototyping, testing, discarding, all in the hopes that we were failing forward towards the right solution. Arriving at the final working solution was all the sweeter when we were told multiple outside engineering teams weren’t able to do what we had done together with Cook.
The Outcome
The resulting design has been awarded several international patents and has continued to inform Cook Medical’s product and IP development, underscoring their competitiveness for years to come.
With significant competing technical, operational and safety demands, Cook’s endovascular graft delivery device proved to be one of CMD’s most ergonomically and mechanical challenging designs, and ultimately one of our most satisfying endeavours.